PubChem API in Python#
by Avery Fernandez
PubChem API Documentation: https://pubchemdocs.ncbi.nlm.nih.gov/programmatic-access
These recipe examples were tested on May 16, 2022.
Attribution: This tutorial was adapted from supporting information in:
Scalfani, V. F.; Ralph, S. C. Alshaikh, A. A.; Bara, J. E. Programmatic Compilation of Chemical Data and Literature From PubChem Using Matlab. Chemical Engineering Education, 2020, 54, 230. https://doi.org/10.18260/2-1-370.660-115508 and vfscalfani/MATLAB-cheminformatics)
Setup#
First, import libraries:
import requests
from pprint import pprint
from time import sleep
Define the PubChem PUG-REST API base URL:
api = 'https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/'
1. PubChem Similarity#
Get compound image#
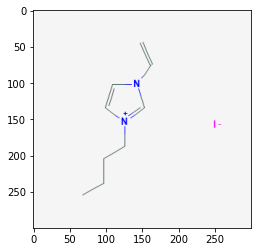
We can search for a compound and display an image, for example: 1-Butyl-3-methyl-imidazolium; CID = 2734162
# Request PNG from PubChem and save file
compoundID = "2734162"
img = requests.get(api + '/cid/' + compoundID + "/PNG").content
with open("2734162.png", "wb") as out:
out.write(img)
# Display compound PNG with Matplotlib
import matplotlib.pyplot as plt
import matplotlib.image as mpimg
img = mpimg.imread('2734162.png')
plt.imshow(img)
plt.show()
Retrieve InChI and SMILES#
request = requests.get(api + 'cid/' + compoundID + '/property/inchi,IsomericSMILES/JSON').json()
pprint(request)
{'PropertyTable': {'Properties': [{'CID': 2734162,
'InChI': 'InChI=1S/C8H15N2/c1-3-4-5-10-7-6-9(2)8-10/h6-8H,3-5H2,1-2H3/q+1',
'IsomericSMILES': 'CCCCN1C=C[N+](=C1)C'}]}}
# Extract InChI
request["PropertyTable"]["Properties"][0]["InChI"]
'InChI=1S/C8H15N2/c1-3-4-5-10-7-6-9(2)8-10/h6-8H,3-5H2,1-2H3/q+1'
# Extract Isomeric SMILES
request["PropertyTable"]["Properties"][0]["IsomericSMILES"]
'CCCCN1C=C[N+](=C1)C'
Perform a Similarity Search#
We will use the PubChem API to perform a Fingerprint Tanimoto Similarity Search (SS).
(2D Tanimoto threshold 95% to 1-Butyl-3-methyl-imidazolium; CID = 2734162)
api = 'https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/'
request = requests.get(api + "fastsimilarity_2d/cid/" + compoundID + "/cids/JSON?Threshold=95").json()
idList = request["IdentifierList"]["CID"]
In the above request value, you can adjust to the desired Tanimoto threshold (i.e., 97, 90, etc.)
len(idList)
297
# display first 25
idList[0:25]
[529334,
304622,
118785,
61347,
12971008,
11448496,
11424151,
11171745,
11160028,
2734236,
2734162,
2734161,
11245926,
53384410,
11788435,
5245884,
2734168,
139254006,
91983981,
87560886,
87559770,
11448364,
10537570,
10154187,
141109628]
Retrieve Identifier and Property Data#
Get the following data for the retrieved CIDs (idList): InChI, Isomeric SMILES, MW, Heavy Atom Count, Rotable Bond Count, and Charge
api = 'https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/'
compoundDictionary = []
for cid in idList:
request = requests.get(api + 'cid/' + str(cid) + "/property/InChI,IsomericSMILES,MolecularWeight,HeavyAtomCount,RotatableBondCount,Charge/JSON").json()
compoundDictionary.append(request["PropertyTable"]["Properties"][0])
sleep(1)
len(compoundDictionary)
297
pprint(compoundDictionary[0:5])
[{'CID': 529334,
'Charge': 0,
'HeavyAtomCount': 10,
'InChI': 'InChI=1S/C8H14N2/c1-2-3-4-6-10-7-5-9-8-10/h5,7-8H,2-4,6H2,1H3',
'IsomericSMILES': 'CCCCCN1C=CN=C1',
'MolecularWeight': '138.21',
'RotatableBondCount': 4},
{'CID': 304622,
'Charge': 0,
'HeavyAtomCount': 10,
'InChI': 'InChI=1S/C8H14N2/c1-3-4-6-10-7-5-9-8(10)2/h5,7H,3-4,6H2,1-2H3',
'IsomericSMILES': 'CCCCN1C=CN=C1C',
'MolecularWeight': '138.21',
'RotatableBondCount': 3},
{'CID': 118785,
'Charge': 0,
'HeavyAtomCount': 8,
'InChI': 'InChI=1S/C6H10N2/c1-2-4-8-5-3-7-6-8/h3,5-6H,2,4H2,1H3',
'IsomericSMILES': 'CCCN1C=CN=C1',
'MolecularWeight': '110.16',
'RotatableBondCount': 2},
{'CID': 61347,
'Charge': 0,
'HeavyAtomCount': 9,
'InChI': 'InChI=1S/C7H12N2/c1-2-3-5-9-6-4-8-7-9/h4,6-7H,2-3,5H2,1H3',
'IsomericSMILES': 'CCCCN1C=CN=C1',
'MolecularWeight': '124.18',
'RotatableBondCount': 3},
{'CID': 12971008,
'Charge': 0,
'HeavyAtomCount': 10,
'InChI': 'InChI=1S/C7H13N2.HI/c1-3-4-9-6-5-8(2)7-9;/h5-7H,3-4H2,1-2H3;1H/q+1;/p-1',
'IsomericSMILES': 'CCCN1C=C[N+](=C1)C.[I-]',
'MolecularWeight': '252.10',
'RotatableBondCount': 2}]
Data Table#
We can display the dictionary as a data table, but we will only do this for the first 25:
# numbers in print statement indicate amount of space used
print ("{:<10} {:<8} {:<16} {:<35} {:<40} {:<18} {:<4} ".format("CID", "Charge", "HeavyAtomCount", "InChI", "IsomericSMILES", "MolecularWeight", "RotatableBondCount"))
for compound in compoundDictionary[0:25]:
cid = compound["CID"]
charge = compound["Charge"]
heavyAtom = compound["HeavyAtomCount"]
inchi = compound["InChI"][0:30] + "..." # only display first 30 characters of InChI
isomeric = compound["IsomericSMILES"]
molecular = compound["MolecularWeight"]
rotatable = compound["RotatableBondCount"]
print ("{:<10} {:<8} {:<16} {:<35} {:<40} {:<18} {:<4} ".format(cid, charge, heavyAtom, inchi, isomeric, molecular, rotatable))
CID Charge HeavyAtomCount InChI IsomericSMILES MolecularWeight RotatableBondCount
529334 0 10 InChI=1S/C8H14N2/c1-2-3-4-6-10... CCCCCN1C=CN=C1 138.21 4
304622 0 10 InChI=1S/C8H14N2/c1-3-4-6-10-7... CCCCN1C=CN=C1C 138.21 3
118785 0 8 InChI=1S/C6H10N2/c1-2-4-8-5-3-... CCCN1C=CN=C1 110.16 2
61347 0 9 InChI=1S/C7H12N2/c1-2-3-5-9-6-... CCCCN1C=CN=C1 124.18 3
12971008 0 10 InChI=1S/C7H13N2.HI/c1-3-4-9-6... CCCN1C=C[N+](=C1)C.[I-] 252.10 2
11448496 0 11 InChI=1S/C8H15N2.HI/c1-3-4-5-1... CCCCN1C=C[N+](=C1)C.[I-] 266.12 3
11424151 0 13 InChI=1S/C8H15N2.CHNS/c1-3-4-5... CCCCN1C=C[N+](=C1)C.C(#N)[S-] 197.30 3
11171745 0 15 InChI=1S/C8H15N2.C2N3/c1-3-4-5... CCCCN1C=C[N+](=C1)C.C(=[N-])=NC#N 205.26 3
11160028 0 10 InChI=1S/C7H13N2.BrH/c1-3-4-9-... CCCN1C=C[N+](=C1)C.[Br-] 205.10 2
2734236 0 11 InChI=1S/C8H15N2.BrH/c1-3-4-5-... CCCCN1C=C[N+](=C1)C.[Br-] 219.12 3
2734162 1 10 InChI=1S/C8H15N2/c1-3-4-5-10-7... CCCCN1C=C[N+](=C1)C 139.22 3
2734161 0 11 InChI=1S/C8H15N2.ClH/c1-3-4-5-... CCCCN1C=C[N+](=C1)C.[Cl-] 174.67 3
11245926 0 13 InChI=1S/C8H15N2.Br2.BrH/c1-3-... CCCCN1C=C[N+](=C1)C.[Br-].BrBr 378.93 3
53384410 0 13 InChI=1S/C8H15N2.Br3/c1-3-4-5-... CCCCN1C=C[N+](=C1)C.Br[Br-]Br 378.93 3
11788435 0 11 InChI=1S/C8H15N2.H2O/c1-3-4-5-... CCCCN1C=C[N+](=C1)C.[OH-] 156.23 3
5245884 1 9 InChI=1S/C7H13N2/c1-3-4-9-6-5-... CCCN1C=C[N+](=C1)C 125.19 2
2734168 1 11 InChI=1S/C9H17N2/c1-4-5-6-11-8... CCCCN1C=C[N+](=C1C)C 153.24 3
139254006 0 12 InChI=1S/C9H15N2.HI/c1-3-5-6-1... CCCC[N+]1=CN(C=C1)C=C.[I-] 278.13 4
91983981 -2 13 InChI=1S/C8H15N2.3BrH/c1-3-4-5... CCCCN1C=C[N+](=C1)C.[Br-].[Br-].[Br-] 378.93 3
87560886 0 12 InChI=1S/C9H15N2.BrH/c1-3-5-6-... CCCC[N+]1=CN(C=C1)C=C.[Br-] 231.13 4
87559770 0 12 InChI=1S/C9H15N2.ClH/c1-3-5-6-... CCCC[N+]1=CN(C=C1)C=C.[Cl-] 186.68 4
11448364 0 14 InChI=1S/C11H21N2.BrH/c1-3-5-7... CCCCN1C=C[N+](=C1)CCCC.[Br-] 261.20 6
10537570 1 11 InChI=1S/C9H17N2/c1-3-4-5-6-11... CCCCCN1C=C[N+](=C1)C 153.24 4
10154187 0 10 InChI=1S/C7H13N2.ClH/c1-3-4-9-... CCCN1C=C[N+](=C1)C.[Cl-] 160.64 2
141109628 0 10 InChI=1S/C7H11FN2/c1-2-3-5-10-... CCCCN1C=CN=C1F 142.17 3
Retrieve Images of Compounds from Similarity Search#
# we will only do this for the first five:
api = "https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/"
for cid in idList[0:5]:
request = requests.get(api + "cid/" + str(cid) + "/PNG").content
sleep(1)
with open(str(cid) + ".png", "wb") as out:
out.write(request)
pprint(cid)
img = mpimg.imread(str(cid) + ".png")
plt.imshow(img)
plt.show()
529334
304622
118785
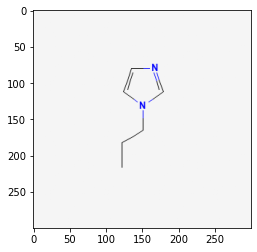
61347
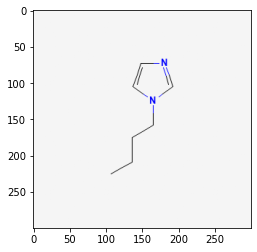
12971008
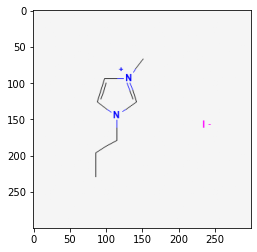
2. PubChem SMARTS Search#
Search for chemical structures from a SMARTS substructure query.
Define SMARTS queries#
View pattern syntax at: https://smartsview.zbh.uni-hamburg.de/
Note: These are vinyl imidazolium substructure searches
smartsQ = ["[CR0H2][n+]1[cH1][cH1]n([CR0H1]=[CR0H2])[cH1]1","[CR0H2][n+]1[cH1][cH1]n([CR0H2][CR0H1]=[CR0H2])[cH1]1","[CR0H2][n+]1[cH1][cH1]n([CR0H2][CR0H2][CR0H1]=[CR0H2])[cH1]1"]
Add your own SMARTS queries to customize. You can add as many as desired within a list
Perform a SMARTS query search#
api = "https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/"
combinedList = []
for smarts in smartsQ:
request = requests.get(api + "fastsubstructure/smarts/" + smarts + "/cids/JSON").json()
combinedList += request["IdentifierList"]["CID"]
sleep(1)
len(combinedList)
830
pprint(combinedList[0:25]) # display 25
[121235111,
86657882,
46178576,
24766550,
139254006,
132274871,
129853306,
129853221,
129850195,
87560886,
87559770,
87327009,
59435292,
141478618,
141176071,
139241369,
138404213,
138373746,
135377330,
135361018,
132427329,
132275640,
129862663,
129850437,
129850146]
Retrieve Identifier and Property Data#
smartsList = []
for cid in combinedList[0:5]: # demo for first 5 CIDs
request = requests.get(api + "cid/" + str(cid) + "/property/InChI,CanonicalSMILES,MolecularWeight,IUPACName,HeavyAtomCount,CovalentUnitCount,Charge/JSON").json()
smartsList.append(request["PropertyTable"]["Properties"][0])
sleep(1)
pprint(smartsList[0:5])
[{'CID': 121235111,
'CanonicalSMILES': 'CC[N+]1=CN(C=C1)C=C.C(F)(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F',
'Charge': 0,
'CovalentUnitCount': 2,
'HeavyAtomCount': 24,
'IUPACName': 'bis(trifluoromethylsulfonyl)azanide;1-ethenyl-3-ethylimidazol-3-ium',
'InChI': 'InChI=1S/C7H11N2.C2F6NO4S2/c1-3-8-5-6-9(4-2)7-8;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h3,5-7H,1,4H2,2H3;/q+1;-1',
'MolecularWeight': '403.3'},
{'CID': 86657882,
'CanonicalSMILES': 'CCCCCCCC[N+]1=CN(C=C1)C=C.[Br-]',
'Charge': 0,
'CovalentUnitCount': 2,
'HeavyAtomCount': 16,
'IUPACName': '1-ethenyl-3-octylimidazol-3-ium;bromide',
'InChI': 'InChI=1S/C13H23N2.BrH/c1-3-5-6-7-8-9-10-15-12-11-14(4-2)13-15;/h4,11-13H,2-3,5-10H2,1H3;1H/q+1;/p-1',
'MolecularWeight': '287.24'},
{'CID': 46178576,
'CanonicalSMILES': 'CCCCCCCCCCCCCCCC[N+]1=CN(C=C1)C=C.[Br-]',
'Charge': 0,
'CovalentUnitCount': 2,
'HeavyAtomCount': 24,
'IUPACName': '1-ethenyl-3-hexadecylimidazol-3-ium;bromide',
'InChI': 'InChI=1S/C21H39N2.BrH/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23-20-19-22(4-2)21-23;/h4,19-21H,2-3,5-18H2,1H3;1H/q+1;/p-1',
'MolecularWeight': '399.5'},
{'CID': 24766550,
'CanonicalSMILES': 'CCCC[N+]1=CN(C=C1)C=C.C(F)(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F',
'Charge': 0,
'CovalentUnitCount': 2,
'HeavyAtomCount': 26,
'IUPACName': 'bis(trifluoromethylsulfonyl)azanide;1-butyl-3-ethenylimidazol-1-ium',
'InChI': 'InChI=1S/C9H15N2.C2F6NO4S2/c1-3-5-6-11-8-7-10(4-2)9-11;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h4,7-9H,2-3,5-6H2,1H3;/q+1;-1',
'MolecularWeight': '431.4'},
{'CID': 139254006,
'CanonicalSMILES': 'CCCC[N+]1=CN(C=C1)C=C.[I-]',
'Charge': 0,
'CovalentUnitCount': 2,
'HeavyAtomCount': 12,
'IUPACName': '1-butyl-3-ethenylimidazol-1-ium;iodide',
'InChI': 'InChI=1S/C9H15N2.HI/c1-3-5-6-11-8-7-10(4-2)9-11;/h4,7-9H,2-3,5-6H2,1H3;1H/q+1;/p-1',
'MolecularWeight': '278.13'}]
Retrieve Images of CID Compounds from SMARTS Search#
api = "https://pubchem.ncbi.nlm.nih.gov/rest/pug/compound/"
for cid in combinedList[0:5]:
request = requests.get(api + "cid/" + str(cid) + "/PNG").content
sleep(1)
with open(str(cid) + ".png", "wb") as out:
out.write(request)
pprint(cid)
img = mpimg.imread(str(cid) + ".png")
plt.imshow(img)
plt.show()
121235111
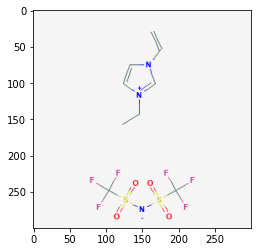
86657882
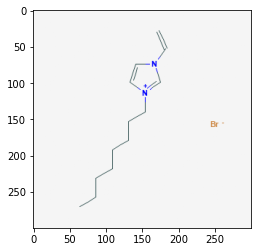
46178576
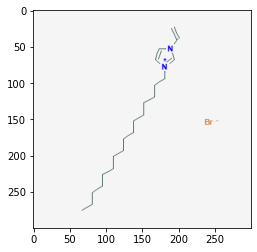
24766550
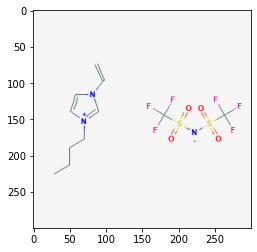
139254006